FDA to Remove Pharma Reps From Advisory Committees, But Conflicts of Interest Persist
After decades of influence—is the damage already done?
This article originally appeared on The Defender and was republished with permission.
Guest post by Brenda Baletti, Ph.D.
The FDA plans to remove employees of pharmaceutical companies that the agency regulates from its advisory commissions and “elevate” the role of patients and caregivers, Commissioner Marty Makary announced Thursday.
The U.S. Food and Drug Administration (FDA) plans to remove employees of pharmaceutical companies that the agency regulates from its advisory commissions and “elevate” the role of patients and caregivers, Commissioner Marty Makary announced Thursday.
Makary, in a post on X, said that historically the agency has been unduly influenced by industry. Although the FDA should partner with industry to ensure a “user-friendly review process,” he said, “the scientific evaluation of new products should be independent.”
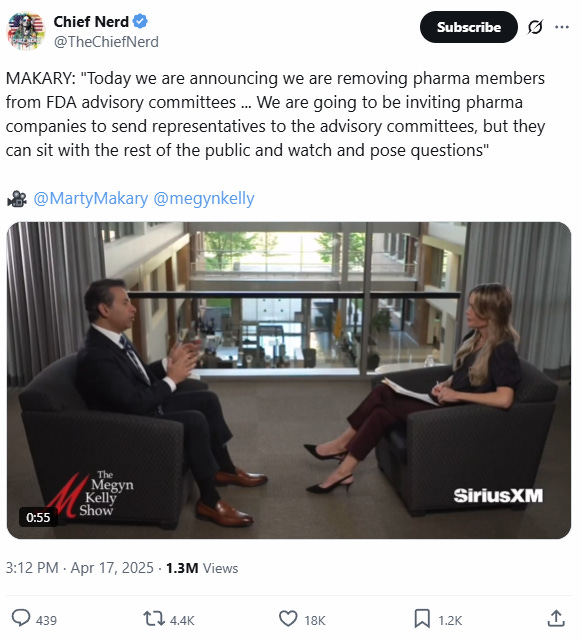
Makary announced the news in an interview with SiriusXM’s Megyn Kelly. He said representatives of pharmaceutical companies would be invited to attend meetings in the same way as the rest of the public — they can sit and ask questions.
The FDA said in a press release that the move is intended “to boost public trust in its decisions and improve how its advisory committees operate.”
Exceptions may be made in rare circumstances when a particular expertise is needed or when pharma reps are required by statute to serve on committees, the agency said.
STAT News reported that the FDA Modernization Act of 1997 requires that non-voting industry reps sit on advisory panels, although that rule appears to be limited to only certain panels.
The FDA convenes advisory panels, intended to be comprised of experts in relevant fields, to provide feedback and recommendations on products under review. The agency does not have to accept advisory panels’ recommendations, although it typically does.
The Big Pharma reps on advisory committees don’t vote on committee decisions.
Earlier this year, mainstream media called foul when the FDA canceled the meeting of its vaccine advisory committee. The vaccine advisory committee for the Centers for Disease Control and Prevention (CDC) was also initially postponed, although it went forward this week.
When the meeting was postponed, Politico reported that U.S. Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. plans to replace members of HHS advisory committees who have conflicts of interest as part of his broader effort to intervene in Big Pharma’s undue influence over regulatory agencies.
Conflicts of interest persist on committees, even if industry reps are removed
Makary’s announcement may be a move in this direction. However, many members of advisory committees at the FDA and other agencies under HHS have major conflicts of interest, even if they are not directly employed by industry — an issue Kennedy flagged as a problem in a February interview with Fox News.
For example, Dr. Paul Offit, who invented a rotavirus vaccine later developed by the pharmaceutical giant Merck, sits on the FDA’s vaccine advisory committee. His hospital, which owns the patent, licenses it to Merck, according to The New York Times.
In another example, KFF Health News reported in April 2024 that 10 of the 14 doctors on an FDA advisory panel reviewing a heart device had received money from or conducted research funded by Abbott, the company that made the device.
A Defender investigation into the new members appointed to the CDC’s vaccine advisory committee last year found that all nine new members named to the committee have financial ties to pharmaceutical companies or have worked with public health agencies to promote the COVID-19, RSV (respiratory syncytial virus), or HPV vaccines.
Another Defender investigation in 2021 found that all members of the FDA advisory committee that authorized the COVID-19 vaccines had ties to pharma.
None of them are direct industry employees.
Open Payments, a government database, records some financial relationships between doctors and certain other healthcare providers and the makers of drugs and medical devices. The data on Open Payments is not comprehensive, but it does offer a window into conflicts of interest among current FDA vaccine advisory committee members.
For example, on the FDA’s current vaccine advisory committee:
Dr. Anna Durbin has received consulting fees from Merck.
Dr. Haley Gans has received consulting fees from Pfizer.
Dr. Arnold Monto has received consulting fees from several pharmaceutical companies, including F. Hoffmann-La Roche, Seqirus USA, Sanofi Pasteur and others.
Dr. Michael Nelson has taken consulting fees from Kaleo Inc.
Dr. Stanley Perlman took research money from Eli Lilly.
Dr. Flor Munoz-Rivas has received hundreds of thousands of dollars in consulting fees from Moderna, Seqirus, Sanofi, AstraZeneca — and nearly $9 million in research funding from Moderna and others.
Dr. Saad B. Omer, another committee member, isn’t listed on Open Payments, but his own research website indicates he has funding from medical industry giants like Kaiser Permanente and major pharma funders, including the Bill & Melinda Gates Foundation.
All of these members are voting members of the committee.
A report on Kelly’s website highlighted another major problem at the FDA — the revolving door.
For example, Dr. Curtis Wright, the FDA’s medical review officer who alone approved the opioid OxyContin — effectively launching the opioid crisis — later went to work for the drug’s maker, Purdue Pharma.
In another example, Dr. Patrizia Cavazzoni left Pfizer to become the FDA’s top drug regulator as director of the Center for Drug Evaluation and Research. In January, she left the FDA. Three weeks later, Pfizer announced that it had hired Cavazzoni to replace its outgoing chief medical officer.
Dr. Scott Gottlieb, FDA commissioner from 2017 through 2019 during the first Trump administration, is now a member of Pfizer’s board of directors.
Related articles in The Defender
FDA Calls Off Meeting to Select Flu Strains for Next Season’s Vaccine
Is Kennedy’s HHS Preparing to Shake Up CDC’s Vaccine Advisory Committee?
17 Pharma Henchmen Who Voted to Experiment on Your Kids — and How to Shun Them
9 New ‘Independent’ Advisers to CDC Publicly Promoted Vaccines or Took Money From Pharma — or Both
Donate to Children’s Health Defense